Ultrasonic Solutions That Truly Work for Silver
Browse Volume:30 Classify:Support
Silver is both beautiful and delicate. Its luster and shine have made it a favorite for jewelry, tableware, coins, and decorative objects for centuries. However, silver also has a frustrating trait: it tarnishes quickly. This tarnish is not dirt. It is a chemical reaction between silver and sulfur-containing compounds in the air, often forming silver sulfide. The result is a darkened, dull surface that hides silver’s natural brilliance.
Cleaning silver can be challenging. Scrubbing with a cloth can help, but only on accessible surfaces. Intricate jewelry, filigree, or antique cutlery often have grooves and engravings that trap particles or tarnish. Regular polishing compounds may be too abrasive for softer silver alloys, and repeated friction wears away fine details over time.
This is why many professionals and collectors turn to ultrasonic cleaning. It is fast, non-abrasive, and can restore tarnished silver without harming its intricate craftsmanship. However, even ultrasonic cleaning requires the right approach, especially when it comes to the cleaning solution used.
How Ultrasonic Cleaners Remove Tarnish and Grime
Ultrasonic cleaning technology relies on high-frequency sound waves, typically ranging between 20 – 200 khz, which are sent through a tank filled with liquid. These sound waves generate tiny vacuum bubbles in the liquid, which then rapidly collapse. This phenomenon is called cavitation. When these bubbles collapse near a solid surface, they release energy that lifts away grime, oils, oxides, and even tarnish from that surface.
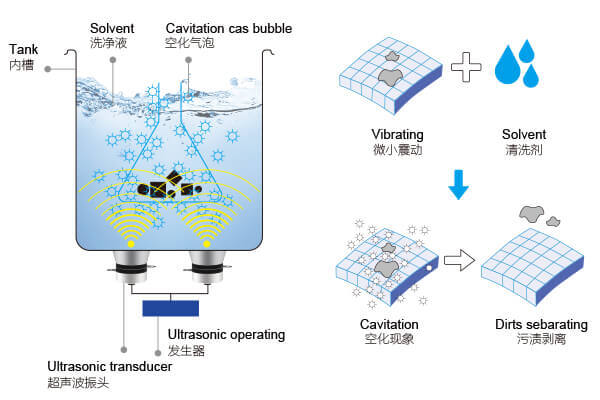
The Principle Behind Ultrasonic Cleaning
Unlike mechanical scrubbing, which only reaches exposed areas, cavitation occurs uniformly throughout the solution. This allows ultrasonic cleaners to clean complex objects evenly, including hard-to-reach crevices. The process is gentle on surfaces yet powerful against stubborn buildup.
For silver, this method is especially useful. Cavitation can break apart the layer of silver sulfide responsible for tarnish, restoring the bright finish beneath. It
works best when paired with a cleaning solution that supports the cleaning process chemically and physically.
The Role of Cleaning Solution in Ultrasonic Systems
The cleaning solution used in an ultrasonic cleaner is not just a carrier for sound waves. It actively influences whether the cavitation process works well, and whether contaminants like silver sulfide can be effectively removed. Water alone can carry sound waves, but it lacks the chemical assistance needed to lift certain types of debris or tarnish.
The cleaning solution reduces surface tension, which allows cavitation bubbles to form more easily and collapse with greater energy. It also contains agents that can dissolve oils, break down metal oxides, and suspend removed particles so they do not reattach to the surface.
When cleaning silver, the wrong type of solution can hinder these processes. For example, using only water may produce weak cavitation. Using dish soap might create foam that absorbs the ultrasonic energy and prevents effective cleaning. Worse still, some detergents can leave behind a film or chemically interact with silver in a way that promotes further tarnishing.
A well-formulated ultrasonic cleaning solution for silver must balance several properties. It must enhance cavitation without foaming. It must include chemical agents that target silver sulfide while being gentle on silver’s surface. And it must rinse away cleanly, leaving no residue.

Silver jewelry cleaning
What Happens When You Use the Wrong Solution
Not every cleaning liquid belongs in an ultrasonic tank. When the wrong solution is used, the outcome is often worse than doing nothing at all. This is especially true for silver, a metal that is both reactive and delicate.
Many people assume that dishwashing liquid, because it cleans well in the kitchen sink, will work the same way in an ultrasonic bath. This assumption causes problems. Dish soap is designed for hand washing. It contains surfactants that break down grease, along with foaming agents, fragrances, and colorants. While effective in the sink, these ingredients behave differently in an ultrasonic environment.
The most immediate issue is foaming. Foam interferes with the ultrasonic process by absorbing and scattering the sound waves that drive cavitation. When foam accumulates in the tank, it reduces the energy transmitted through the solution. This weakens the bubbles that are needed to dislodge contaminants. Cleaning becomes slower and less thorough, especially in recessed or intricate areas.
Another problem is chemical residue. Dish soaps often leave behind a film, which may not be noticeable when rinsed from glass or ceramic but can cling to the surface of metals like silver. Over time, this film can attract more dirt or even react with the environment to accelerate tarnish.
In some cases, dishwashing detergents contain ingredients that are mildly alkaline or acidic. These may slowly etch the surface of silver, especially if the piece is silver-plated. The damage may not be visible after a single cleaning, but repeated exposure can dull the finish or cause pitting.
When used in an ultrasonic tank, the wrong solution turns the process from precision cleaning into uncontrolled chemistry. That is why selecting a solution specifically formulated for silver is critical.
Key Characteristics of an Effective Silver Cleaning Solution
An ultrasonic cleaning solution for silver must do more than just clean. It must protect, preserve, and prepare the surface for long-term resistance to tarnish. To do this, the solution requires a carefully balanced formula.
First, the solution should maintain a near-neutral pH, typically between 7.5 and 9. This range is gentle on silver and effective at lifting tarnish. Solutions that are too acidic or too alkaline may remove tarnish but damage the metal in the process. Silver-plated items are particularly vulnerable to harsh chemicals, which can erode the thin surface layer and expose the base metal beneath.
Second, the formula must include a mild chelating agent or oxidizing component that can react with silver sulfide, breaking it down into water-soluble compounds. Sodium citrate and ammonium carbonate are commonly used in silver-safe solutions because they are effective against tarnish and safe for repeated use.
A good solution also contains a non-foaming surfactant. This helps lower surface tension and allows cavitation bubbles to form properly, without introducing foam. It should also contain a dispersant to keep loosened particles suspended in the solution, preventing them from resettling onto the item during the cleaning cycle.
Finally, anti-tarnish agents may be included. These substances form a protective layer on the surface of clean silver. While not permanent, this coating can slow the formation of new tarnish, giving the silver a longer-lasting shine between cleanings.
All of these characteristics must be present in a well-designed ultrasonic cleaning solution for silver. Missing even one can lead to subpar results, and using a general-purpose or homemade alternative rarely meets all of these criteria.

Ultrasonic cleaning agent
Why Ordinary Dishwashing Detergent Fails
Dishwashing liquid is one of the most common cleaning agents in households. It is safe for skin, effective on grease, and smells pleasant. However, it is not suitable for ultrasonic cleaning, particularly when it comes to cleaning silver.
The first issue is foaming. Most dish soaps are formulated to create lather. While this is desirable in a sink, it is a serious problem in an ultrasonic cleaner. Foam acts as a cushion that absorbs ultrasonic energy, reducing the intensity of cavitation. The result is weaker bubbles and less effective cleaning. In some cases, excessive foam can overflow from the tank or interfere with the device’s heating and frequency controls.
The second issue is surface residue. Dishwashing detergents are designed to rinse off glass and plastic with a steady stream of water. But in ultrasonic cleaning, where items are often submerged for several minutes, the detergent can cling to metal surfaces. This residue can attract dirt and moisture, accelerating future tarnish rather than preventing it.
Chemical composition is the third concern. Dish soaps contain a blend of surfactants, colorants, perfumes, stabilizers, and sometimes enzymes. These ingredients are not selected with silver in mind. Some may react with silver ions, while others may leave trace compounds that degrade the finish. Even when no immediate damage occurs, repeated exposure over multiple cleaning cycles can dull the surface or increase susceptibility to future tarnish.
Lastly, dish soap does not contain any agents that specifically target silver sulfide. It is not designed to break down tarnish, nor to protect precious metals. In the ultrasonic tank, this makes it a passive medium at best, and a harmful one at worst.
When people report poor results from ultrasonic cleaning, they are often using dish soap or another incompatible solution. The machine itself is not the problem; the chemistry is. Only a dedicated, silver-compatible solution can deliver the deep cleaning and long-term protection that ultrasonic cleaning is capable of providing.
Safe and Unsafe Ingredients for Silver in Ultrasonic Baths
Choosing a cleaning solution for ultrasonic use is not only about what works, but also about what is safe. Silver is sensitive to both high acidity and strong alkalinity. Certain chemicals can brighten silver surfaces, but others may cause damage, especially to silver plating, antique finishes, or items with mixed materials.
Safe ingredients include:
- Sodium citrate: A gentle chelating agent that binds with silver sulfide and makes it easier to remove tarnish without harming the silver beneath.
- Ammonium carbonate: Common in commercial silver dips and also found in some ultrasonic-compatible cleaners. It is effective at breaking down oxidation without pitting the surface.
- Non-ionic surfactants: These reduce surface tension and improve cavitation without creating foam.
- Deionized water: Helps avoid mineral residues or ionic interactions that could discolor silver during cleaning.
- Corrosion inhibitors: These form a temporary protective film to reduce re-tarnishing after cleaning.
Unsafe ingredients to avoid:
- Chlorine bleach: Highly corrosive and can permanently damage silver.
- Acetic acid (vinegar): Too acidic for prolonged exposure, especially in ultrasonic agitation. It may etch surfaces or weaken solder joints.
- Sodium hypochlorite: Found in some household cleaners, this oxidizer is dangerous to both silver and human health in enclosed ultrasonic systems.
- Strong alkalis: Sodium hydroxide or potassium hydroxide can strip surfaces or cause blackening.
Even natural or DIY ingredients often recommended online may carry risks. Lemon juice and baking soda, for example, may react unpredictably when heated or exposed to cavitation. In ultrasonic cleaning, these reactions can intensify, creating pitting or unwanted chemical residues.
A silver-safe solution must strike a careful balance. It should have enough strength to react with tarnish, but not so much that it endangers the finish. Professional products are tested for this balance. Home remedies rarely are.
Comparing Home Remedies and Professional Formulas
Home cleaning methods for silver have existed for centuries. Baking soda paste, aluminum foil baths, vinegar soaks, and lemon juice are just a few examples. While these may remove surface tarnish under certain conditions, they are poorly suited to ultrasonic cleaning.
Take the baking soda and aluminum foil method, for instance. It works by creating an electrochemical reaction that transfers tarnish from silver to aluminum. This is effective for some flatware or coins but does not work well in ultrasonic tanks. The reaction is not improved by cavitation, and the presence of aluminum can interfere with the ultrasonic process.
Vinegar is another common home remedy. Although it has some tarnish-removing capability, its high acidity can etch the silver, especially under ultrasonic agitation. When heated in an ultrasonic tank, vinegar can release fumes and increase corrosion risk, particularly to joints or mixed-metal items.
Dishwashing detergent, as discussed earlier, lacks the chemical agents needed to remove silver sulfide. It contributes foam and residue without enhancing the cleaning power of the machine.
In contrast, professional ultrasonic cleaning solutions are designed specifically for use with cavitation. They are tested to ensure that the surfactants, pH level, and active ingredients work in harmony. Most commercial silver cleaners used in ultrasonic applications have passed corrosion and material compatibility tests. They also rinse clean, reducing the risk of re-tarnishing and buildup.
The performance difference is not minor. In controlled tests, silver jewelry cleaned with a professional solution shows more consistent brightness, less residue, and longer tarnish resistance compared to jewelry cleaned with a home-mixed solution. While homemade methods may seem appealing, especially from a cost perspective, they often lead to repeated cleanings or irreversible damage.
Use Cases: Jewelry, Utensils, and Antique Silver
Silver comes in many forms, and each type of object presents unique cleaning challenges. Jewelry, for example, may contain stones, adhesives, or mixed metals that must be considered before ultrasonic cleaning. Utensils may have engraved handles, hollow spaces, or welds that require special care. Antiques often come with patinas that should not be removed entirely.
Silver jewelry benefits greatly from ultrasonic cleaning, especially when designed without glued stones. Tarnish and skin oils can be removed evenly, restoring shine and detail. When paired with the correct cleaning solution, even detailed filigree pieces come out looking brilliant.
Utensils and silverware, such as forks, spoons, and serving pieces, often carry both visible tarnish and invisible contaminants like oils, salts, or food residues. Ultrasonic cleaning removes these thoroughly, but the solution must be able to dissolve both organic and inorganic matter. A professional formula ensures that no residues remain in engraved logos or handle joints.
Antique silver poses the greatest risk for cleaning. Many collectors appreciate the aged patina that gives antiques their character. Over-cleaning or using the wrong solution can strip this layer, reducing the item’s value. In ultrasonic applications, the cleaning time and solution strength must be adjusted carefully. Using a silver-specific cleaner with a short cleaning cycle allows the removal of unwanted tarnish while preserving the visual and historical depth of the piece.
In all cases, rinsing and drying after cleaning are essential. Any leftover solution, no matter how mild, can continue reacting with silver over time. The use of deionized water for rinsing and soft cloths for drying helps maintain a clean, even surface.
Commercial Silver Cleaning Solutions: What Professionals Use
In jewelry repair shops, watch workshops, and antique restoration labs, ultrasonic cleaning is a daily routine. Professionals in these environments do not rely on guesswork. They choose commercial-grade solutions that are specifically formulated for silver, and they do so for good reason.
A typical professional ultrasonic solution for silver includes a balanced formula of pH stabilizers, non-foaming surfactants, and tarnish-removal agents. These products undergo compatibility testing across different silver alloys, including sterling silver, coin silver, and silver-plated items. The goal is to maximize cleaning efficiency while preserving fine detail and preventing micro-abrasions.
For example, jewelry manufacturers often use solutions that include ammonium carbonate and specialized wetting agents. These components react with silver sulfide without harming soldered joints or embedded materials. In laboratories handling antique cutlery or coins, more neutral pH solutions are used to avoid altering surfaces that have aged naturally over decades.
Another reason professionals prefer commercial products is consistency. With a tested solution, the results are predictable. There is no guesswork about the impact on different finishes, and the cleaning process can be standardized across batches of items. For businesses that handle dozens or even hundreds of silver objects daily, this level of control is essential.
Furthermore, many professional solutions are biodegradable and non-toxic, meeting workplace safety standards. They also come with manufacturer recommendations for ultrasonic frequency, solution concentration, and optimal temperature, allowing technicians to fine-tune their cleaning protocols.
The takeaway is simple: when quality, safety, and repeatability matter, professionals choose ultrasonic solutions that are designed for silver from the start. For serious cleaning tasks, these solutions offer far more than convenience; they offer confidence.
Do-It-Yourself Cautions and Best Practices
While commercial solutions are the best option for most applications, some users prefer to mix their own ultrasonic cleaning formulas at home. In limited cases, this can work, provided it is done carefully and with proper materials. However, it carries risks that should not be overlooked.
One common DIY mistake is using kitchen-grade acids or alkalis, such as vinegar or ammonia. These substances may remove tarnish, but they can also damage the silver’s surface when subjected to ultrasonic agitation. The vibrations in the tank intensify chemical reactions, so what seems gentle in the sink can become harmful in a cleaner.
Another risk is imprecise measurement. Unlike commercial formulas, which are pre-balanced, homemade mixtures often rely on estimation. Adding too much of an active ingredient, or failing to neutralize a reaction properly, can result in corrosion or discoloration.
There are, however, some safe DIY practices. Using a mild detergent that is free from fragrances and dyes, mixed with deionized water, can serve as a general-purpose cleaner for lightly soiled silver. It will not remove tarnish, but it can help eliminate surface oils or residues. Rinsing thoroughly after cleaning is important, especially when using any homemade solution.
Temperature control is also crucial. Many DIY users overlook this detail. Heating the solution to around 40 degrees Celsius improves cavitation and dissolving power. However, exceeding 50 degrees Celsius can cause chemical changes or soften adhesives used in jewelry, leading to long-term damage.
If one chooses to go the DIY route, it is essential to research each ingredient, start with minimal exposure time, and test on a small, less valuable item first. Even with these precautions, the cleaning results may not match those achieved with a professionally formulated solution.
The Influence of Frequency and Temperature
Ultrasonic cleaning is not a one-size-fits-all process. The effectiveness of any cleaning solution, including those used for silver, is directly affected by the frequency and temperature of the ultrasonic bath. Getting these two factors right is essential for safe and effective results.
Frequency determines the size and intensity of the cavitation bubbles. Lower frequencies, such as 25 or 28 kilohertz, produce larger bubbles with greater energy. These are ideal for removing heavy dirt or polishing compounds but can be too aggressive for delicate silver pieces. Higher frequencies, such as 40 or 68 kilohertz, create smaller bubbles that clean more gently. For fine silver, especially jewelry or thin-plated items, a frequency of 40 kilohertz is generally recommended.
Temperature influences both the chemical reaction rate and the physical cavitation process. As temperature increases, surface tension decreases, which allows more effective bubble formation. Most silver cleaning solutions perform best in a range of 35 to 45 degrees Celsius. At this temperature, tarnish removal is accelerated without risking chemical overreaction or heat damage.
However, higher temperatures can also increase the volatility of some chemicals and weaken adhesive bonds in assembled items. Overheating can darken silver or cause residues to bake onto the surface, rather than rinse away cleanly.
Because solution performance depends on both heat and frequency, many commercial cleaning products provide recommendations for optimal use. Ignoring these can result in either incomplete cleaning or irreversible surface changes.
By matching the right solution with the appropriate frequency and temperature, users can achieve a deep clean that preserves the beauty of silver, rather than jeopardizing it.
Post-Cleaning Care: Rinsing, Drying, and Tarnish Prevention
Cleaning silver in an ultrasonic tank is only part of the care process. What happens after the cleaning cycle ends is just as important. Even when using a high-quality solution, failure to rinse or dry properly can compromise the results and shorten the time before tarnish returns.
After cleaning, silver should be removed from the tank promptly and rinsed with clean, deionized water. This step is crucial. Rinsing removes any dissolved contaminants and prevents them from redepositing onto the item. Tap water can be used in a pinch, but it often contains minerals that leave spots or react with silver surfaces. Deionized or distilled water eliminates that risk.
The drying process matters too. Silver should never be left to air dry in a humid environment, as moisture can accelerate oxidation. Instead, items should be dried immediately with a soft, lint-free cloth. For small crevices or chains, a burst of compressed air can help remove water trapped in difficult spots.
Some users choose to apply a tarnish inhibitor or store cleaned silver in anti-tarnish pouches. These extra steps can be worthwhile, especially in regions with high humidity or air pollution. In professional environments, silver is often handled with gloves after cleaning to avoid reintroducing oils or moisture from skin contact.
Ultimately, the effectiveness of ultrasonic cleaning depends not only on the machine and solution used but also on how the silver is handled afterward. Proper rinsing and drying preserve the work done during cleaning and help maintain brightness for much longer.
Eco-Friendly and Non-Toxic Alternatives
Environmental awareness has grown significantly in recent years, and ultrasonic cleaning has evolved to reflect that. Many modern silver cleaning solutions are now formulated to be biodegradable, phosphate-free, and non-toxic, without compromising effectiveness.
These green solutions often use plant-based surfactants and pH-neutral chemistry. Some include natural chelating agents derived from citric acid or other organic sources. While they may work slightly more slowly than aggressive chemical blends, they provide a safer option for home users and environmentally regulated workplaces.
Disposal is another important consideration. Spent ultrasonic cleaning solution can contain dissolved metals, surfactants, and other compounds that should not be poured down the drain. Even eco-friendly solutions can accumulate contaminants that require proper disposal. Users should check local guidelines for hazardous waste disposal, especially if cleaning is done frequently or in large volumes.
Reusability can reduce waste. Many commercial solutions are designed to last through multiple cleaning cycles before they lose effectiveness. Filtering systems can extend life by removing suspended particles, and heating only when necessary helps preserve active ingredients.
For users looking to balance performance with responsibility, selecting a green-certified solution and following proper disposal practices is the best approach. It ensures clean silver and a cleaner environment at the same time.
Expert Opinions: Jewelers, Conservators, and Chemists Speak
Across the silver care industry, professionals consistently emphasize one principle: the cleaning solution matters as much as the ultrasonic machine itself.
Jewelry repair specialists often warn against household soaps and home remedies. One master jeweler, with over two decades of experience, noted that ultrasonic cleaning with dish detergent tends to “cloud high-polish finishes and leave a film that dulls the appearance.” He recommends only using branded, pH-balanced solutions specifically tested for silver.
Conservators who handle museum-grade artifacts are even more cautious. In restoration labs, ultrasonic cleaning is used sparingly and only with products that have passed metal compatibility assessments. One conservator explained that a silver-plated goblet cleaned with the wrong detergent lost part of its decorative layer due to improper pH control and agitation speed.
Chemists who develop cleaning solutions for industrial and laboratory use point to the science behind product formulation. According to one formulation chemist, “A silver-safe solution is about more than cleaning power. It’s about how molecules interact with silver ions under heat and vibration. You cannot guess your way to that balance in a kitchen.”
Their combined insights underscore what testing also shows: ultrasonic cleaning is a powerful tool, but only when paired with the right chemistry. Without that balance, even expensive machines cannot produce consistent or safe results.
Final Thoughts: Choose Intelligently for Your Silver’s Future
Ultrasonic cleaning is one of the most effective ways to care for silver, especially when precision and preservation matter. But the machine alone is not enough. The solution inside the tank determines whether the process enhances your silver’s appearance or unintentionally harms it.
Ordinary dishwashing detergent, though convenient, is not designed for use in ultrasonic environments or on precious metals. Its ingredients can reduce cavitation, introduce foam, and leave behind residues that attract more tarnish. Worse still, repeated use can slowly degrade the surface of fine or plated silver.
By contrast, a properly formulated ultrasonic cleaning solution for silver supports the cleaning process chemically and physically. It removes tarnish without abrasion, rinses away cleanly, and protects the surface for longer-lasting shine. Professionals in jewelry, conservation, and industrial fields rely on these solutions for their predictability and performance. Home users benefit from the same level of care when they choose these products.
The difference between dull and brilliant silver often comes down to what goes in the tank. Choosing wisely means preserving beauty, value, and craftsmanship, item after item, cycle after cycle.
 Granbo Sonic
Granbo Sonic














